Biography
Anton read Natural Sciences at Trinity College Dublin, specialising in Genetics before coming to the University of Cambridge for his Ph.D. studies in 1998, with Prof. Christos Ouzounis, working on protein families and biological network analysis. His postdoctoral work was with Prof. Chris Sander at Memorial Sloan-Kettering Cancer Center, New York working on small RNA target prediction of regulatory sites in mRNAs. In 2004 he started his own independent laboratory at the Wellcome Trust Sanger Institute, exploring the functions of small non-coding RNAs in various biological processes and diseases. In 2004 he moved to the EMBL - European Bioinformatics Institute, as a Group Leader where he continued to work on non-coding RNAs, including piwi-associated RNAs, lncRNAs and RNA modifications. His laboratory moved to the Department of Pathology in 2018. Anton is also academic lead for the genomics facility at the department which specialises in large-scale sequencing, microarrays and genotyping projects. Anton oversees the Oxford Nanopore facility that hosts the School of Biological Sciences PromethION device.
Research
Complete genome sequencing projects have generated enormous amounts of data. Although progress has been rapid, a significant proportion of the genes in any given genome are either unannotated or possess a poorly characterised function. Our group aims to detect, predict and describe the functions of genes, proteins, mRNAs and regulatory non-coding RNAs as well as their interactions in living organisms and their implications for disease. We are a multi-disciplinary team using wet-lab experimental techniques, computational biology and high-throughput genomics to solve these problems. Our work encompasses a number of areas:
The epitranscriptome - RNA modification and methylation
Gene regulation is a fundamental process that underpins cell development, differentiation, function and homeostasis. The discovery of epigenetic marks on both DNA and histones heralded a new era in our understanding of gene regulation. We believe that we are on the cusp of another wave of critical biological discoveries regarding similar marks placed on mRNAs and regulatory RNAs. These events sculpt the transcriptome altering how mRNAs are processed, spliced, translated, degraded and how they interact with proteins and other RNAs. Understanding this fundamental process is of critical importance to further our understanding of biological regulation and understanding mechanisms that underlie disease aetiology and susceptibility.
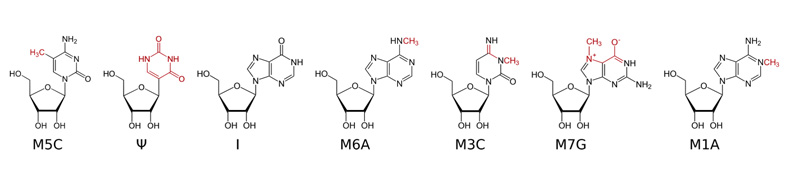
Methylated Nucleosides in RNA.
Enright Lab student sequencing an RNA library directly using Nanopore sequencing
To fully explore this growing world of epitranscriptomic modifications and their effects, we are combining cutting-edge genomics techniques including Illumina high-throughput sequencing, Oxford Nanopore Direct RNA sequencing and single nucleotide mass-spectrometry to try and detect RNA modifications. Over the coming years we will be developing novel experimental protocols, algorithms and methods for detecting and describing RNA modifications and their impact in living systems.
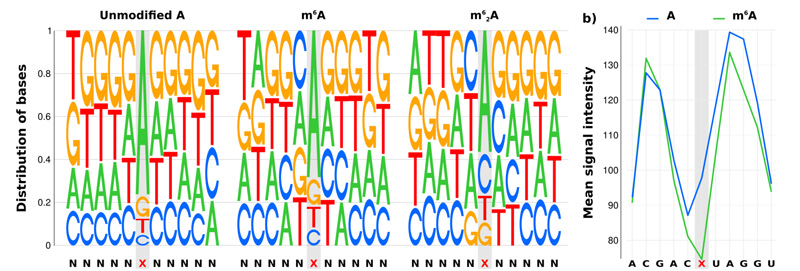
Oxford Nanopore direct RNA sequencing of modified nucleotides in RNA.
Algorithms and methods for non-coding RNA research
We have been working for over 20 years to understand how small RNAs such as microRNAs (miRNAs) regulate transcription and mRNA stability. We developed one of the first algorithms for miRNA target detection (miRanda) and worked with miRBase to make the first computational miRNA targets available to the community. The advent of next-generation sequencing made the detection of miRNAs and their global, transcriptome-wide effects, far easier to detect and allowed us to work directly in experimental systems where we could directly measure the effects of microRNAs in model organisms and human disease. We also developed a range of algorithms and web-based analysis platforms to aid the community involved in this field.
Additionally, we worked extensively on other classes of non-coding RNA molecules such as piwi-RNAs and long non-coding RNAs. Recently, we explored how processing of small RNA sequencing datasets allows the detection of 3' modification events such as uridylation and Adenosine to Inosine RNA editing.
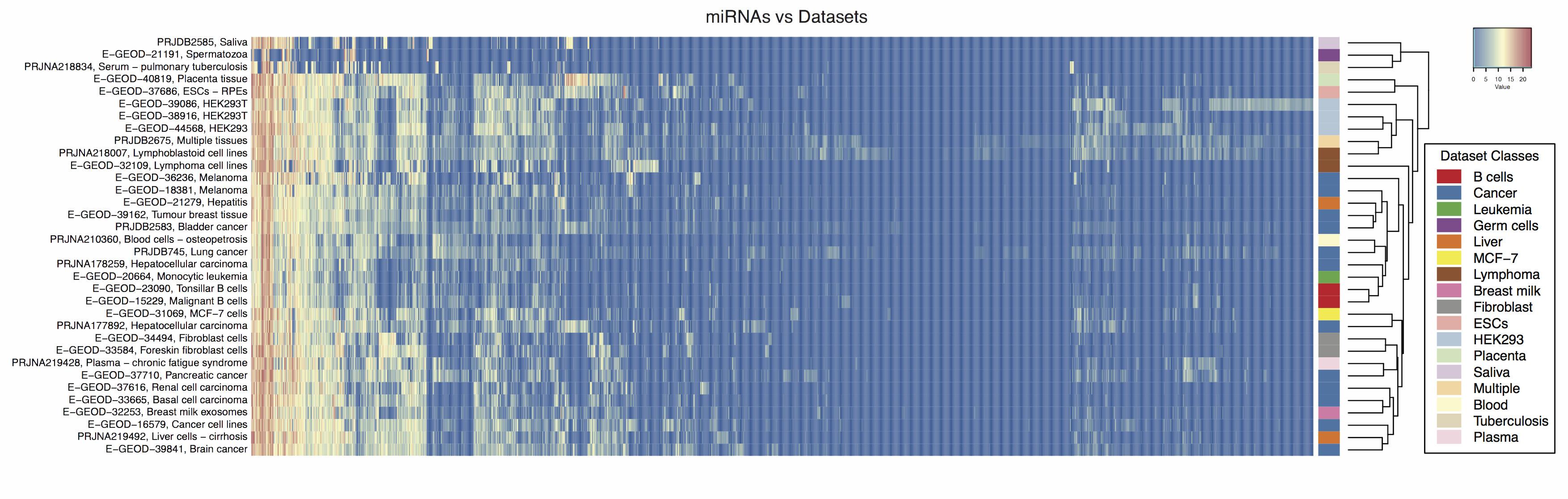
Modification profiles for microRNAs present in human cancer datasets detected using ChimiRa.
Elucidating the functions of non-coding RNAs in biological processes and disease
We have worked on the roles of miRNAs and lncRNAs in a variety of biological systems and disease models. Our algorithm Sylamer has been widely used to assess the effects of miRNAs on transcriptome level data obtained from sequencing in model organisms, cell lines and patient samples. This has allowed us to establish roles for a number of miRNAs with our colleagues and collaborating laboratories including: Maternal-Zygotic transition, Mouse models of deafness, Ichtyosis and a range of cancers. We work closely with Profs. Matthew Murray and Nicholas Coleman on the roles of miRNAs in paediatric germ cell tumours and on HPV pathogenesis.
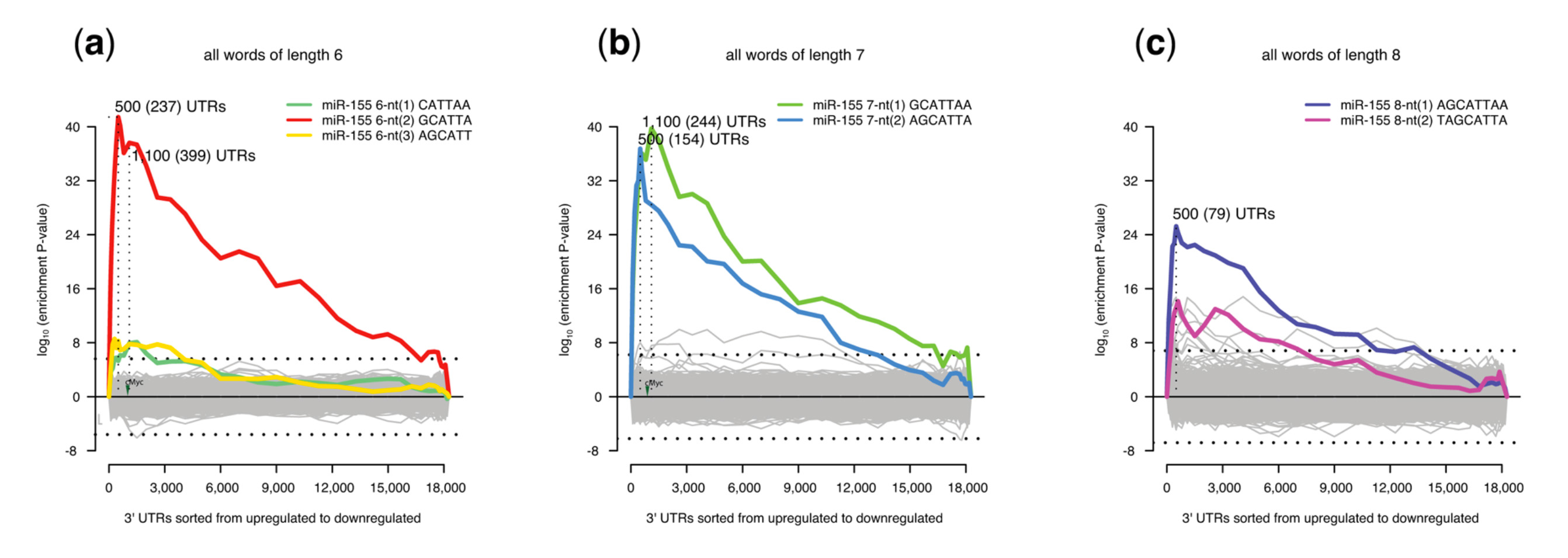
Sylamer motif enrichment profiles showing microRNA motifs present in T-helper cells from mice deficient in miR-155
Machine Learning and Data Visualisation in biology
We have an ongoing interest in the analysis of large datasets using machine learning techniques such as SVMs, RVMs, unsupervised clustering (e.g. Markov Clustering), Random Forests and Logistic Regression. Recently, we released miRNovo, a machine learning microRNA classifier for accurate prediction of miRNAs from large-scale sequencing projects. We have developed a number of tools for large-scale visualisation of biological networks including the BioLayout tool developed with BBSRC funding in collaboration with Prof. Tom Freeman (University of Edinburgh).
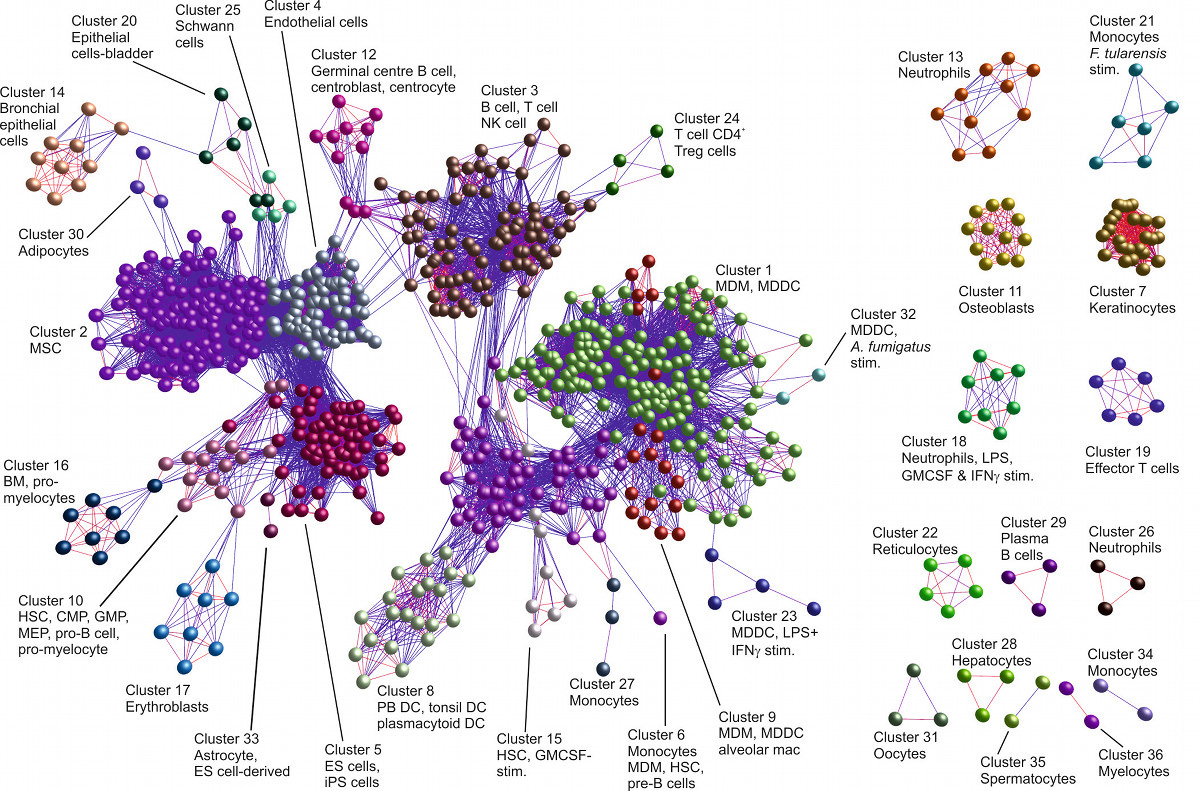
BioLayout Express 3D visualisation of samples from a human cell atlas (Tom Freeman) based on Markov Clustering (MCL)
We welcome visitors to the laboratory who wish to undertake their own experimental, computational or combined analyses under our guidance. We are always happy to talk to prospective students, visitors and lab members.
Publications
Recent Selected Publications
- Vitsios DM, Kentepozidou E, Quintais L, Benito-Gutiérrez E, van Dongen S, Davis MP*, Enright AJ*. Mirnovo: genome-free prediction of microRNAs from small RNA sequencing data and single-cells using decision forests. Nucleic Acids Res., 2017 gkx836
- Ivanova I, Much C, Di Giacomo M, Azzi C, Morgan M, Moreira PN, Monahan J, Carrieri C, Enright AJ*, O'Carroll D* The RNA m6A Reader YTHDF2 Is Essential for the Post-transcriptional Regulation of the Maternal Transcriptome and Oocyte Competence, Mol Cell. 2017 Sep 21;67(6):1059-1067.
- Morgan M, Much C, DiGiacomo M, Azzi C, Ivanova I, Vitsios DM, Pistolic J, Collier P, Moreira PN, Benes V, Enright AJ, O'Carroll D. mRNA 3' uridylation and poly(A) tail length sculpt the mammalian maternal transcriptome. Nature. 2017 Aug 17;548(7667):347-351
- Davis MP, Carrieri C, Saini HK, van Dongen S, Leonardi T, Bussotti G, Monahan JM, Auchynnikava T, Bitetti A, Rappsilber J, Allshire RC, Shkumatava A, O'Carroll D, Enright AJ. Transposon-driven transcription is a conserved feature of vertebrate spermatogenesis and transcript evolution. EMBO Rep. 2017 Jul;18(7):1231-1247
- Vitsios DM, Davis MP, van Dongen S, Enright AJ. Large-scale analysis of microRNA expression, epi-transcriptomic features and biogenesis. Nucleic Acids Res. 2017 Feb 17;45(3):1079-1090
- Bussotti G, Leonardi T, Clark MB, Mercer TR, Crawford J, Malquori L, Notredame C, Dinger ME, Mattick JS, Enright AJ. Improved definition of the mouse transcriptome via targeted RNA sequencing. Genome Res. 2016 May;26(5):705-16. doi: 10.1101/gr.199760.115.
- Vitsios DM, Enright AJ. Chimira: analysis of small RNA sequencing data and microRNA modifications. Bioinformatics. 2015 Oct 15;31(20):3365-7. doi: 10.1093/bioinformatics/btv380. Epub 2015 Jun 20.
Enright lab members underlined
* denotes co-corresponding or co-first authors
All Publications
Teaching and Supervisions
- Part II Pathology - Genetics and Genomics of Disease - Option Organiser and Lecturer
- Part 1B - Mathematical and Computational Biology - Module Organiser and Lecturer
Supervisions: 1A Biology of Cells, 1B Biology of Disease, Part 1B Mathematical and Computational Biology. Part II Pathology
Lab Members
We are a multi-disciplinary team of both wet, dry and combined biologists.
Ph.D. Students
- Albert Kang
- Stephanie Wenlock
- Melanie Maranian
- Sethlina Aryee
Masters Students and Undergraduates
- Alexandra Ioana
- Arinjoy Bannerjee
- Kate Zielinski
- Sam Carling
- Adam Agbamu
- Levia Yee
Other Professional Activities
- External Examiner: Royal College of Surgeons in Ireland - Advanced Therapeutic Technologies
- Fellow (Pathology), Director of Studies and Deputy Graduate Tutor - Trinity Hall, Cambridge
- Academic Lead - Genomics (Cambridge Genomics Services), Dept. of Pathology, Cambridge



