Submitted by Livia Harriman on Mon, 30/06/2025 - 11:55
A recent study published by the Koronakis Lab has revealed an unexpected finding.
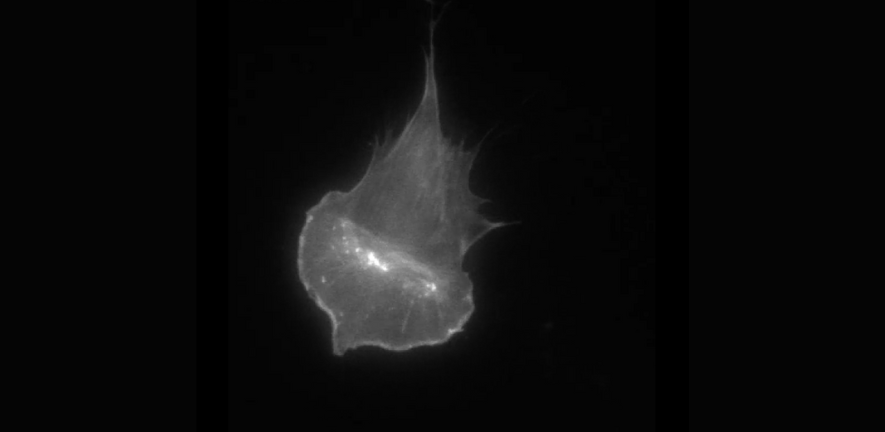
We usually think of proteins as little machines that need to be “switched on” to work. But in our recent study, we found something unexpected—some proteins can still affect cell behaviour even when they’re turned off. PAK proteins regulate how cells grow, move, and die, and influence the spread of cancer cells throughout the body. All these activities result from the effect of PAK proteins on the actin cytoskeleton, a cellular structure that helps give the cell its shape and determine its overall morphology.
When PAKs are inhibited with a drug (G5555) in mouse cells (movie) the internal structure of the cells reorganised in surprising ways. We observed the accumulation of a signalling molecule, PIP3, on the bottom side of the cells, which caused reorganisation of the actin cytoskeleton and affected their movement. Further investigation revealed that even when PAKs aren’t active, simply having them and their partners present in large amounts affects the amount of PIP3 produced by another enzyme, PI3K.
Our work demonstrates that the unexpected activity of an inhibited protein has implications for the development of cancer treatments that target PAK proteins.
Link to the Article
A role for class I p21-activated kinases in the regulation of the excitability of the actin cytoskeleton J Cell Sci. 2025 Jun 15;138(12
Tyler JJ, Davidson A, Poxon ME, Llanses Martinez M, Hume P, King JS, Koronakis V.
Link to the Research Highlight
UnPAKing kinase roles: catalytic-dependent and -independent functions

