Open Access DOI: https://doi.org/10.1016/j.celrep.2019.09.074
MHC class I molecules play a critical role in immunosurveillance by presenting antigenic peptides to CD8+ T lymphocytes. Research in the Boyle group in the Department of Pathology focuses on understanding how peptides are selected on MHC class I for immune recognition. Over recent years, they have demonstrated that a protein called TAPBPR functions as an intracellular peptide editor, which shapes the peptide repertoire displayed on MHC class I molecules for immune recognition (1). In 2018, work led by Tudor Ilca, a PhD student in the group, revealed recombinant TAPBPR can be utilised to load immunogenic peptides of choice directly onto plasma membrane expressed MHC class I (2).
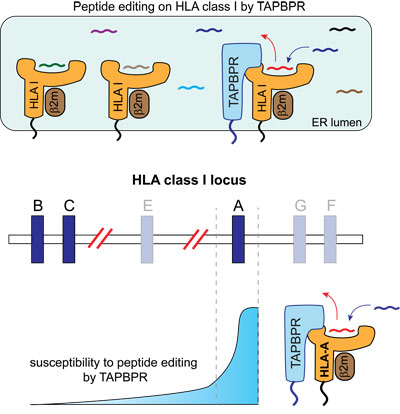
Despite MHC class I being the most polymorphic proteins found in humans, very little is known about how variation in these molecules influence their interaction with molecular chaperones. In a new study, Ilca et al., perform the first large scale and unbiased study to explore how polymorphisms in MHC class I influence their ability to interact with TAPBPR (3). They reveal a striking preference of TAPBPR for HLA-A molecules, particularly for members of the A2 and A24 superfamilies, over HLA-B and -C molecules. Furthermore, they discovered that molecular features of the MHC class I F pocket were responsible for the increased propensity of HLA-A molecules to undergo peptide editing by TAPBPR. Even subtle polymorphisms in the F pocket of specific HLA-B molecules linked to disease altered the level of peptide editing mediated by TAPBPR.
Given that peptide selection likely plays an important role linking the association of specific MHC class I molecules to infectious diseases and autoinflammatory conditions, these findings may offer new insight regarding how apparently subtle variation in MHC class I can have a significant impact on susceptibility to disease.
- Hermann C, van Hateren A, Trautwein N, Neerincx A, Duriez PJ, Stevanovic S, et al. TAPBPR alters MHC class I peptide presentation by functioning as a peptide exchange catalyst. Elife. 2015;4:e09617.
- Ilca FT, Neerincx A, Wills MR, de la Roche M, Boyle LH. Utilizing TAPBPR to promote exogenous peptide loading onto cell surface MHC I molecules. Proc Natl Acad Sci U S A. 2018;115(40):E9353-E61.
- Ilca FT, Drexhage LZ, Brewin E, Peacock S, Boyle LH. Distinct polymorphisms in HLA class I molecules govern their susceptibility to peptide editing by TAPBPR. Cell Reports. 2019.

