 The Koronakis lab has published a new paper in PNAS that sheds light on a key protein trafficking system essential for the survival and pathogenicity of many bacteria.
The Koronakis lab has published a new paper in PNAS that sheds light on a key protein trafficking system essential for the survival and pathogenicity of many bacteria.
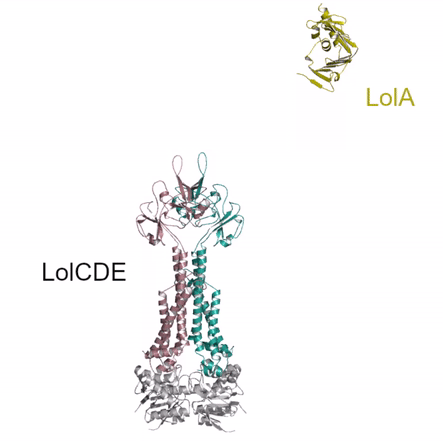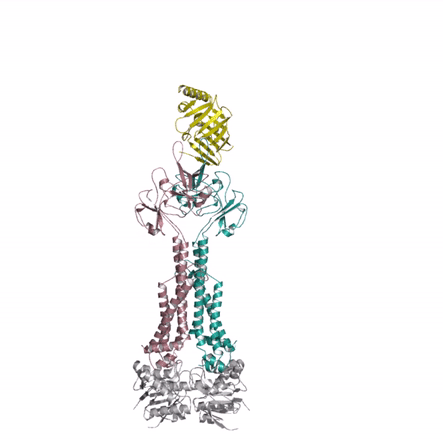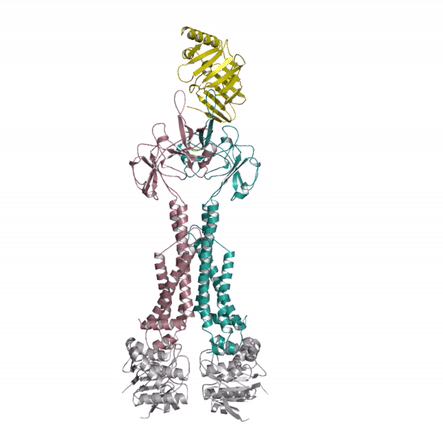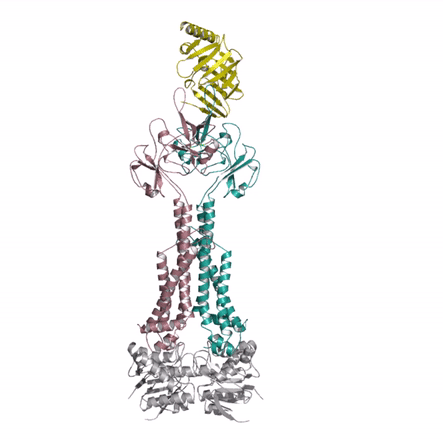
Bacterial lipoproteins are vital structural components of the cell envelope and are responsible for the insertion of lipopolysaccharides and other outer membrane proteins. A dedicated pathway, the Lol system, extracts mature lipoproteins from the inner membrane and transports them to the outer membrane. The authors used X-ray crystallography to investigate a key intermediate in the process demonstrating how the inner membrane complex LolCDE recruits the chaperone, LolA, and primes it to receive lipoprotein substrate. The structural similarity of LolCDE with an antibiotic resistance pump solved by the same authors last year suggests that the LolCDE complex might use the same mechanotransmission mechanism (see movie) to extract the lipoprotein from the inner membrane.
The new structure will aid efforts to design new antibiotics that target this essential system and is part of a wider effort by the Koronakis lab to understand the function of bacterial membrane machineries. The work is supported by funding from the MRC and Wellcome Trust.
Kaplan E, Greene NP, Crow A & Koronakis V (2018). Insights into bacterial lipoprotein trafficking from a structure of LolA bound to the LolC periplasmic domain. Proceedings of the National Academy of Sciences

