Published in PNAS: Structure and mechanotransmission mechanism of the MacB superfamily.
Researchers from the Pathology department at the University of Cambridge have published a ground-breaking new paper that sheds light on how bacteria defend themselves against antibiotics and secrete protein toxins.
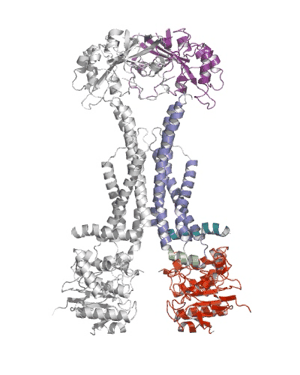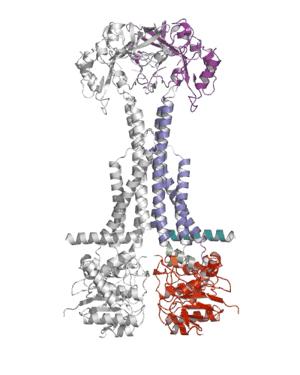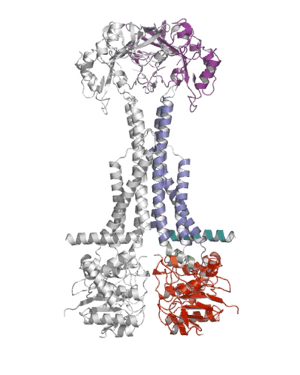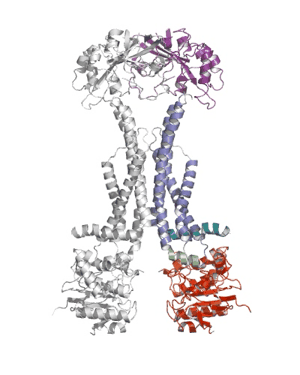
Using X-ray crystallography, a group led by Prof Vassilis Koronakis has revealed the structure of the MacB ABC transporter with bound ATP.
MacB is the powerhouse component of a larger complex of proteins (termed a tripartite efflux pump) that provides bacteria with resistance to antibiotics such as erythromycin, colistin and bacitracin. Pathogens also use this pump to secrete internally-produced protein toxins and virulence factors that help the bacterium to establish infections.
Shape changes in MacB generated by ATP binding and hydrolysis suggest a unique ‘mechanotransmission’ mechanism (see Movie) that is harnessed by the assembled pump to drive substrates out of the bacterium via a pore-like protein in the outer membrane (TolC).
The new work on MacB forms part of a larger drive by the Koronakis lab to understand the role of TolC-related efflux pumps in bacterial antibiotic resistance.
The paper is published in the journal, Proceedings of the National Academy of Sciences.
- Crow, Greene, Kaplan, Koronakis (2017) Structure and mechanotransmission mechanism of the MacB superfamily Proceedings of the National Academy of Sciences

