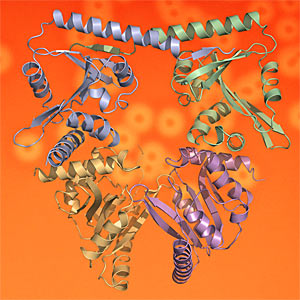
 In this week’s PNAS, Drs Nick Greene and Allister Crow publish their research, led by Professors Vassilis Koronakis and Colin Hughes, which uncovers the high resolution crystal structure of the toxin-activating acyl transferase. By exploring the crystal and solution structures and active site of the virulence enzyme they were able to set out details of the apparently unique biochemical mechanism that activates a family of bacterial toxins - ‘hemolysins’, 'leukotoxins' and ‘cytolysins’ - that subvert, kill and lyse mammalian tissue and immune cells. These toxins are key virulence factors for a number of medically-important pathogens including uropathogenic E. coli (causing urinary tract and kidney infections), enterohemorrhagic E. coli (causing serious food-borne enteric disease) and Bordetella pertussis (the cause of whooping cough).
In this week’s PNAS, Drs Nick Greene and Allister Crow publish their research, led by Professors Vassilis Koronakis and Colin Hughes, which uncovers the high resolution crystal structure of the toxin-activating acyl transferase. By exploring the crystal and solution structures and active site of the virulence enzyme they were able to set out details of the apparently unique biochemical mechanism that activates a family of bacterial toxins - ‘hemolysins’, 'leukotoxins' and ‘cytolysins’ - that subvert, kill and lyse mammalian tissue and immune cells. These toxins are key virulence factors for a number of medically-important pathogens including uropathogenic E. coli (causing urinary tract and kidney infections), enterohemorrhagic E. coli (causing serious food-borne enteric disease) and Bordetella pertussis (the cause of whooping cough).
Greene NP, Crow A, Hughes C, Koronakis V (2015) Structure of a bacterial toxin-activating acyltransferase. Proceedings of the National Academy of Sciences doi: 10.1073/pnas.1503832112
The new work builds on the discovery of the toxin activation mechanism nearly 25 years ago by Vassilis Koronakis and Colin Hughes showing how such pore-forming toxins are matured to the active toxic form by an apparently unique fatty acylation of protoxin internal lysines. Reconstitution of protoxin maturation in vitro revealed that the reaction is directed by a specialized bacterial acyl transferase that uses acyl carrier protein (ACP) as the fatty acid donor.
The new structure-function insights provided by Greene et al provides vital new molecular understanding of the key virulence enzyme and the protein interactions underlying the activation mechanism. This raises the possibility of developing inhibitors of toxin activation that would render the toxin impotent.
Issartel J-P, Koronakis V, Hughes C (1991) Activation of E. coli prohemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature 351:759-61
Stanley P, Packman L, Koronakis V, Hughes C (1994) Fatty acylation of two internal lysine residues required for the toxic activity of E.coli hemolysin. Science 266:1992-96
Stanley P, Koronakis V, Hughes C (1998) Acylation of Escherichia coli hemolysin: a unique protein lipidation mechanism underlying toxin function. Microbiology & Molecular Biology Reviews 62:309-33

