Our Research
Major Histocompatibility Complex (MHC) class I molecules play a fundamental role orchestrating the immune system and inducing protective immune responses against tumours and pathogens. They do this by presenting peptides derived from pathogenic and tumourgenic proteins to the immune system. My laboratory explores the pathway that controls peptide selection onto MHC molecules. This has important translational potential given the importance of MHC molecules in infectious disease, cancer and autoimmunity.
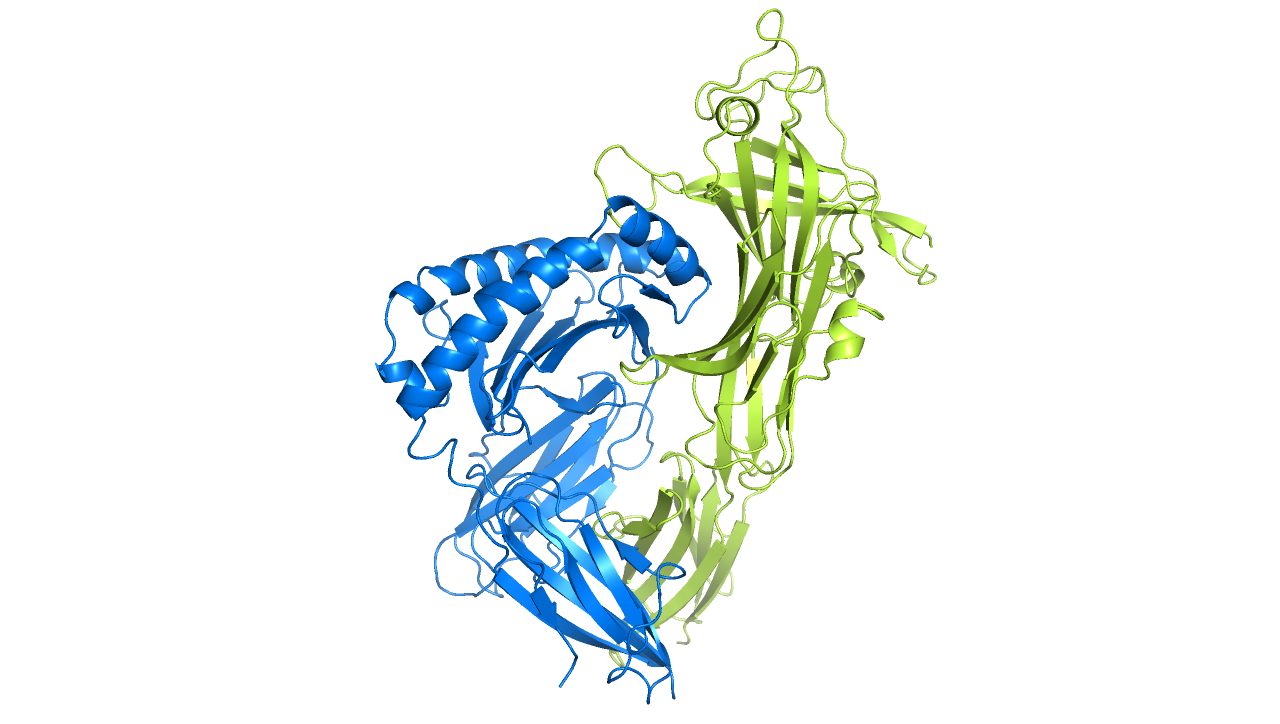
In 2013, we discovered a completely novel MHC class I specific component in the antigen processing and presentation pathway, a protein called TAPBPR. We discovered that TAPBPR is an MHC-I peptide editor that shapes the final antigen repertoire displayed for immune recognition. Additionally, we revealed a loop region of TAPBPR (consisting of residues K22-D35) is involved in mediating peptide dissociation of MHC-I, thus revealing new mechanistic insight into how peptides are selected for immune recognition. Furthermore, we found that by serving as a molecular bridge between peptide-receptive MHC-I and UDP-glucose:glycoprotein glucosyltransferase 1 (UGT1), TAPBPR promotes the glucosylation of the glycan attached on MHC-I, subsequently causing the MHC-I to recycle back to the peptide loading complex.
Although TAPBPR normally functions as an intracellular peptide editor, my laboratory discovered that recombinant TAPBPR can be used to promote peptide exchange directly on plasma-membrane expressed MHC-I. Thus, recombinant TAPBPR can be utilised to decorate cells with immunogenic peptides. This ability to make cells recognisable by the immune system opens up the potential to utilise the function of TAPBPR therapeutically. This might serve as a valuable resource in the fight against cancer.
Key publications
Ilca FT, Boyle LH (2021). The glycosylation status of MHC class I molecules impacts their interactions with TAPBPR. Molecular Immunology. Nov;139:168-176
Ilca FT, Neerincx A, Hermann C, Marcu A, Stevanović S, Deane JE, Boyle LH (2018). TAPBPR mediates peptide dissociation from MHC class I using a leucine lever. eLife. 7:e40126
Ilca FT, Neerincx A, Wills MR, de la Roche M, Boyle LH (2018). Utilizing TAPBPR to promote exogenous peptide loading onto cell surface MHC I molecules. Proc Natl Acad Sci U S A. 115:E9353-E9361
Neerincx A & Boyle LH (2017). Properties of the tapasin homologue TAPBPR. Curr Opin Immunol. 46:97-102.
Neerincx A, Hermann C, Antrobus R, van Hateren A, Cao H, Trautwein N, Stevanović S, Elliott T, Deane JE, Boyle LH (2017). TAPBPR bridges UDP-glucose:glycoprotein glucosyltransferase 1 onto MHC class I to provide quality control in the antigen presentation pathway. eLife. 6:e23049
Hermann C, van Hateren A, Trautwein N, Neerincx A, Duriez PJ, Stevanović S, Trowsdale J, Deane JE, Elliott T, Boyle LH (2015). TAPBPR alters MHC class I peptide presentation by functioning as a peptide exchange catalyst. eLife (2015) 4:e09617
Boyle LH, Hermann C, Boname JM, Porter KM, Patel PA, Burr ML, Duncan LM, Harbour ME, Rhodes DA, Skjødt K, Lehner PJ, Trowsdale J (2013). Tapasin-related protein TAPBPR is an additional component of the MHC class I presentation pathway. Proc Natl Acad Sci U S A. 110:3465-70
For all publications and more on the lab, visit www.teamtapbpr.co.uk or follow us on twitter.
