Submitted by Administrator on Wed, 11/12/2013 - 13:33
18 May 2009
Scientists at the University of Cambridge have uncovered the final piece in the jigsaw revealing the structure of ‘efflux pumps’ which allow Salmonella and other disease-causing bacteria to develop resistance to antibiotics and other drugs. The research, supported by the Wellcome Trust, allows greater understanding of how bacteria escape treatment and may help scientists develop new strategies to prevent antibiotic resistance.
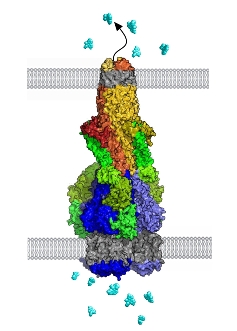
Efflux pumps have evolved as survival mechanisms for the bacteria, reducing the concentration of noxious chemicals within the cells to levels that do not inhibit bacterial functions. These substances include naturally-occurring molecules toxic to the bacteria, such as bile salts in our gut. However, bacteria now also use the pumps to expel many antibiotics and other drugs that we use in the therapy of infections. The efflux pumps can deal with a great many drugs so they are important in the increasing incidence of bacterial multi-drug resistance, which is a growing threat to clinical treatment of infections.
Professors Vassilis Koronakis and Colin Hughes from the Department of Pathology have spent two decades studying the structure and function of these pumps. Now, together with Dr Martyn Simmons, a Cambridge Oppenheimer Research Fellow, the researchers have elucidated the structure of the final component of the pumps, enabling them to see more clearly how the bacteria evade antibiotics and develop resistance.
Salmonella and other so-called 'Gram-negative' bacteria, such as E. coli and Pseudomonas, are bound by two membranes, so the efflux pumps must therefore traverse both membranes in order to pump substances out. Other types of cells, such as human cancer cells and malaria parasites, also have efflux pumps, but these cells only contain a single membrane that the pumps have to cross, making their structure much more simple.
"The challenge for the bacteria is to rid itself of potentially damaging molecules across the unique envelope," says Professor Hughes. "They do this using beautifully simple, yet complex, biological nanomachines."
The bacterial pumps pick up drugs via a transporter in the inner membrane, which delivers them to a "trash chute" known as a TolC exit duct in the outer membrane. A third component - the "adaptor" - connects these two components, opening the TolC exit duct to eject drugs out of the cell. The researchers have now managed to elucidate this whole tripartite structure, which is published in the Proceedings of the National Academy of Sciences.
Professor Hughes suggests that knowing the structure of the bacterial tripartite pumps allows further research to better understand how they work, and presents new possibilities for developing crucial new antibiotics. "This new research shows how the bits come together. Knowing the key components and their assembly can open up new therapeutic targets - in particular by preventing the pumps assembling in the first place."
Read more in:
